Research | Departments
Research
Departments
Research | Departments
Research
Departments

Professor
Cell and Molecular Biology, Cancer Biology, Cancer Stem Cells, Cancer Molecular Medicine, Pancreatic Cancer, Breast Cancer, Cancer Treatment Resistance
Laboratory of Advanced Molecular Therapeutics (AMT Lab)
tsaik@tmu.edu.tw
Dr. Tsai is a rigorously trained physician-scientist focusing on basic and translational cancer research with a career goal aiming at developing breakthrough therapeutics targeting cancer aggressiveness and treatment refractoriness. Dr. Tsai graduated with an M.D. from Taipei Medical University (Taiwan) in 1993, and completed his clinical training in internal medicine, gastroenterology, and GI oncology. Dr. Tsai then earned his Ph.D. in Biological Sciences – Genetics and Complex Diseases from Graduate School of Arts and Sciences, Harvard University (MA, USA) in 2005. During his thesis work, Dr. Tsai established novel 3D coculture models to interrogate stress-induced stromal-epithelial interactions. In 2007, he conducted postdoctoral research at the University of Pennsylvania (PA, USA) and the University of California San Francisco (CA, USA) and spearheaded the study of novel tissue-architecture-related mechanisms of multi-drug resistance in cancer. Following his postdoc research, Dr. Tsai joined the National Institute of Cancer Research in National Health Research Institutes (NHRIs) as an Investigator, and then became the Director and Professor of Graduate Institute of Clinical Medicine at Taipei Medical University. Dr. Tsai is the recipient of many awards, including Harvard Presidential Scholar, USA, Outstanding Research Award, Taiwan, Kobayashi’s Foundation Award, Japan, and Young Scientists Research Achievement Award of NHRIs, Taiwan. He is the first or corresponding author of more than 30 research papers in top-notch biomedical journals such as Nature Cancer, Gastroenterology, J Exp Med, J Am Coll Cardiol, Gut, Cancer Res, etc, and he also served as the PI in several GI cancer-related clinical trials. His major scientific achievements include the identification of a regulatory hub of developmental/stemness signaling and invadopodia that drives cancer stemness and progression, which may hold promises for development into novel CSC-targeted therapies. He co-identified a cell-intrinsic and chromatin-mediated cellular “death checkpoint” whereby most malignant tumors resist cytotoxic stress; the targeting of which yields an exciting opportunity for sensitizing malignant tumors to chemotherapy and immunotherapy.
Research highlights of the AMT Lab
Cancer metastasis and treatment resistance are the two main causes of patient mortality, whereas the underlying cellular and molecular mechanisms are still far from clear. Notwithstanding many decades of basic and clinical research, the therapeutic outcome of cancer patients remains less than satisfactory. The research at the Laboratory of Advanced Molecular Therapeutics (AMT Lab) is aimed at identifying novel and critical regulators of cancer metastasis and treatment resistance that may have important diagnostic and therapeutic implications. Our group uses unique and integrative approaches combining genetics, proteomics, bioinformatics, and tissue engineering principles to study cancer metastasis and its heterogeneity. Our work in these themes has led to the identification of key mediators and novel mechanisms of cancer stemness and metastasis, which provides new insights into cancer aggressiveness. Another line of our research is focused on the role of chemotherapy-treated stroma in tumor progression and therapeutic failure. We have uncovered a paradoxically oncogenic role of chemotherapy-treated stromal cells in pancreatic cancer and breast cancer, and have delineated the underlying signaling pathways and molecular mechanisms. Targeting the therapy-induced stromal-epithelial signaling may be a new avenue for improving the therapeutic outcome of cancer patients. Overall, the main thrusts of the research at the AMT Lab include the following major areas:
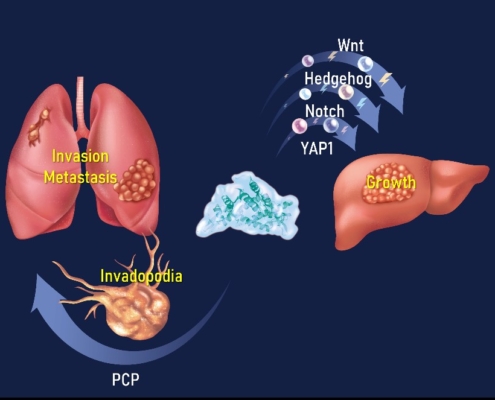
| Figure 1. ASPM as the regulatory hub of cancer growth, invasion, and metastasis (AMT Lab 2023). |
1. Li-Hsin Cheng, Ph.D. (postdoctoral researcher).
2. Hsiao-Yen Hsieh, Ph.D. (postdoctoral researcher).
3. Tai-Yan Liao, M.S. (lab manager).
1. Wang WY, Hsu CC, Wang TY, Li CR, Hou YC, Chu JM, Lee CT, Liu MS, Su JJ, Jian KY, Huang SS, Jiang SS, Shan YS, Lin PW, Shen YY, Lee MT, Chan TS, Chang CC, Chen CH, Chang IS, Lee YL, Chen LT, Tsai KK. A gene expression signature of epithelial tubulogenesis and a role for ASPM in pancreatic tumor progression. Gastroenterology 2013;145:1110-1120 (IF=33.883; corresponding author).
2. Tsai KK, Huang SS, Northey J, Liao WY, Hsu CC, Cheng LH, Werner M, Chuu CP, Chatterjee C, Lakins, JN, Weaver VM*. Screening of organoids derived from patients with breast cancer implicates the repressor NCOR2 in cytotoxic stress response and anti-tumor immunity. Nature Cancer 2022;3:734-752 (IF=23.177; first and co-corresponding author).
3. Cheng LH, Hsu CC, Liao WY, Yang PM, Liao TY, Chan TS, Tsai KK. ASPM activates Hedgehog and Wnt signaling to promote small cell lung cancer stemness and progression. Cancer Research 2023;83:830-844 (IF=13.312; corresponding author).
4. Tsai KK, Bae BI, Hsu CC, Cheng LH, Shaked Y. Oncogenic ASPM is a regulatory hub of developmental and stemness signaling in cancer. Cancer Research 2023;83:2993-3000 (IF=13.312; first and corresponding author).
5. Chan TS, Hsu CC, Pai VC, Liao WY, Huang SS, Tan KT, Yen CJ, Hsu SC, Chen WY, Shan YS, Li CR, Lee MT, Jian KY, Chu JM, Lien GS, Weaver VM, Tsai KK. Metronomic chemotherapy prevents therapy-induced stromal activation and induction of tumor-initiating cells. Journal of Experimental Medicine 2016;213:2967-2988 (IF=17.579; corresponding author

 Total Users : 354216
Total Users : 354216