Research | Departments
Research
Departments
Research | Departments
Research
Departments
Cancer metastasis and treatment resistance are the two main causes of patient mortality, whereas the underlying cellular and molecular mechanisms are still far from clear. Notwithstanding many decades of basic and clinical research, the therapeutic outcome of cancer patients remains less than satisfactory. The research at the Laboratory of Advanced Molecular Therapeutics (AMT Lab) is aimed at identifying novel and critical regulators of cancer metastasis and treatment resistance that may have important diagnostic and therapeutic implications. Our group uses unique and integrative approaches combining genetics, proteomics, bioinformatics, and tissue engineering principles to study cancer metastasis and its heterogeneity. Our work in these themes has led to the identification of key mediators and novel mechanisms of cancer stemness and metastasis, which provides new insights into cancer aggressiveness. Another line of our research is focused on the role of chemotherapy-treated stroma in tumor progression and therapeutic failure. We have uncovered a paradoxically oncogenic role of chemotherapy-treated stromal cells in pancreatic cancer and breast cancer, and have delineated the underlying signaling pathways and molecular mechanisms. Targeting the therapy-induced stromal-epithelial signaling may be a new avenue for improving the therapeutic outcome of cancer patients. Overall, the main thrusts of the research at the AMT Lab include the following major areas:
Increasing evidence suggests that cancer stem cells (CSCs) are the major driving force of cancer dissemination and distant metastasis. It is plausible that therapies targeting the subpopulation of CSCs with high metastasis potentials, the so-called “metastatic CSCs”, may yield potential therapeutic opportunities for metastatic cancer and hold considerable promises for improving patients’ outcomes.
The Wnt pathway plays a crucial role in cancer stemness. Activation of Wnt signaling in CSCs at the distant metastasis sites dictates the successful colonization of the infiltrated cancer cells. The AMT Lab led by Dr. Tsai has identified a novel oncoprotein, ASPM (abnormal spindle-like microcephaly associated), which maintains the oncogenic and invasive potential of CSCs in a wide variety of cancers, including pancreatic, gastric, prostate, lung, and liver cancers. We show that isoform 1 of ASPM specifically augments Wnt signaling by positively regulating Dishevelled proteins in various cancers (Gastroenterology 2013; Oncogene 2018; Journal of Pathology 2019; Cancer Research 2023). Our subsequent works further unraveled the role of ASPM-i1 as a regulatory hub of development-associated signaling pathways, including Wnt, Hedgehog, Notch, and Hippo pathways, in cancer cells and CSCs (Cancer Research 2023). We are currently investigating the role of ASPM-i1 in other cancer biology, such as invadopodia biogenesis, and exploiting its potential utility as a pathway- and stemness-informed prognostic biomarker and therapeutic target in advanced and aggressive cancers.
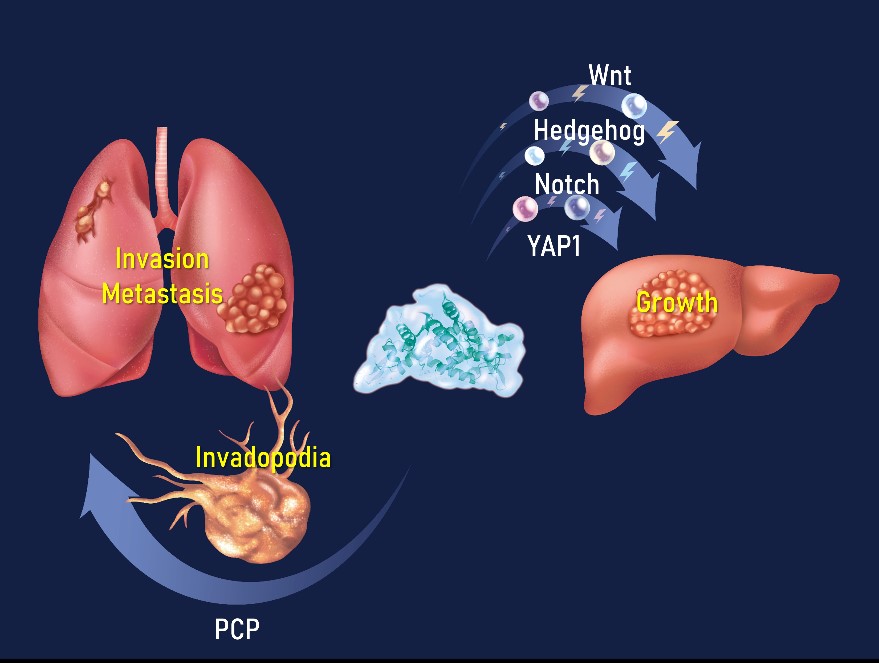
Figure 1. ASPM as the regulatory hub of cancer growth, invasion, and metastasis (AMT Lab 2023).
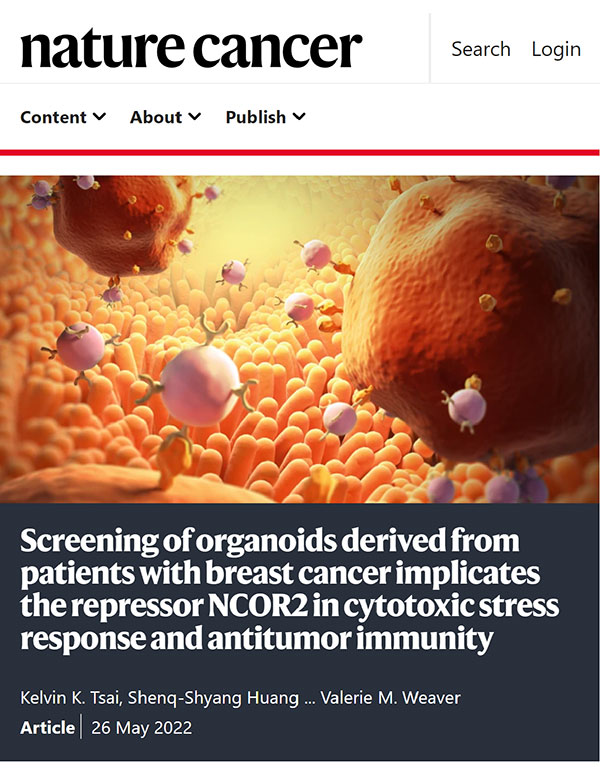
Resistance to anti-tumor treatment contributes to patient mortality. Functional proteomic screening of chemotherapy-treated breast cancer patient-derived organoids (PDOs) identified nuclear co-repressor-2 (NCOR2) histone deacetylase as an inhibitor of cytotoxic stress response and anti-tumor immunity (Nature Cancer 2022). High NCOR2 in the tumors of breast cancer patients predicted chemotherapy refractoriness, tumor recurrence, and poor prognosis. Molecular studies revealed that the NCOR2/HADC3 “Death Checkpoint” inhibits anti-tumor treatment by repressing IRF1-dependent gene expression and interferon (IFN) signaling. Reducing NCOR2 or impeding its epigenetic activity by modifying its interaction with HDAC3 enhanced chemotherapy responsiveness and restored anti-tumor immunity.

We developed a “Decoy of NCOR2” gene therapy, which potentiated chemo-, and immune checkpoint therapy by dismantling the NCOR2 Death Checkpoint, thus permitting transcription of IRF1-regulated pro-apoptosis and inflammatory genes in response to therapies. The discovery underscores the power of using PDOs for anti-tumor drug studies. Targeting stress and inflammatory-repressor complexes such as the NCOR2/HDAC3 Death Checkpoint could overcome treatment resistance and improve cancer patient outcomes (video abstract featured in Research Square: https://www.researchsquare.com/article/rs-3462346/v1).

Email |
Profile | Academic Hub/Pure Experts
Assistant Professor (M.D., Ph.D.)
Immunology, Proteomics, Tuberculosis, Lung diseases
Tuberculosis Research Center, Department of Pulmonary Medicine
Dr Denise was born in Indonesia and graduated as a Medical Doctor from Universitas Gadjah Mada, which is one of the oldest and best medical schools in the country. Working as a medical doctor in a tropical country, she often tended to patients with infectious diseases caused by bacteria, viruses, and parasites. She noticed that these patients showed distinct clinical manifestations and responded differently to treatment.
She worked as a medical researcher in the Tuberculosis Research Center, Wan Fang Hospital up to now. She is also currently appointed as Assistant Professor in the Graduate Institute of Clinical Medicine, Taipei Medical University.
She developed an interest in learning about immunology and proteomics, notably to decipher clinical sample profiles.
It drew her attention and led to her decision to pursue her Master’s degree education in the Tropical Medicine field in 2015, where she learned more specifically about the role of the immune system in disease prevention and/or progression. She finished her Master’s degree in 10 months and was later awarded a scholarship to continue her study in a double-degree Ph.D. program between Universitas Gadjah Mada, Indonesia, and Taipei Medical University, Taiwan.
–
–
Ph.D. (postdoctoral researcher)
Ph.D. (postdoctoral researcher)
M.S. (lab manager)
YT Jheng, DU Putri, MQ Nguyen, HC Chuang, KY Lee, CL Han.
Differential protomic profiles of lung injury in rat models upon pulmonary exposure to air pollution.
European Respiratory Journal 62 (suppl 67)
Abstract
Chronic obstructive pulmonary disease (COPD) is one of the major causes of morbidity and mortality globally. Inhalation of particulate matter (PM) air pollution has been studied to closely associate with COPD. However, the pathogenesis mechanisms underlying PM2.5-induced lung injury is largely unknown, leading to the poor stratification and treatment of the disease. Thus, we aim to explore the underlying molecular mechanisms associated with PM-mediated lung injury by quantitative proteomics analysis of lung tissues from ageing and young rat models with whole body exposure to traffic-related PM pollutants and compared it with that in rat models exposed to high-efficiency particulate air-filtered gaseous pollutants. Our data showed that before lung function decline the 0.5-yr rats had exhibited differential dysregulation of proteins involved in oxidative stress, cellular metabolism, calcium signalling, inflammatory responses, and actin dynamics under exposures to PM and gaseous pollutants. On the contrary, more significant and consistent molecular effects were observed in 1.5-yr rats exposed to PM and gaseous pollutants, of which the malignancy-related ERB signalling pathways were activated additionally in PM-exposed ageing rats. Based on our data, we proposed a detailed pathogenic mechanism to depict temporal and dynamic molecular regulations associated with PM- and gaseous pollutants-induced lung injury. We expect that our findings would provide valuable information towards progression of air pollution-caused lung injury and serve as a repository to search for potential draggable targets.
DU Putri, CK Huang, TY Ou, CF Lin, MC Lee, CS Hung, CH Lee.
Persistent dysregulation of cellular immunity following COVID-19 recovery despite minimal post-COVID-19 sequelae manifestation.
Journal of Infection 86 (5), 486-488
Abstract
No abstract provided.
DU Putri, CL Chen, CH Wang, YM Sue, PC Tseng, CF Lin, CW Tsai, et al.
Hemodialysis acutely altered interferon-gamma release assay test result and immune cell profile.
Journal of Microbiology, Immunology and Infection 55 (2), 332-335
Abstract
Patients receiving hemodialysis (HD) are at risk of TB development. IGRA-positive patients showed significant decrease in quantitative IGRA result with alterations in CD3+CD4+CD45RO+, NK cell, and monocyte subsets immediately upon HD procedure. Our result suggested that the timing of IGRA testing is crucial in end-stage renal disease population.
DU Putri, CF Lin, CS Hung, CK Huang, TY Ou, CY Lai, PC Tseng, et al.
Distinct B and NKT cell responses shape the delayed response to ChAdOx1 nCoV-19 vaccine in end-stage renal disease.
Journal of Infection 84 (6), e122-e125
Abstract
No abstract available.
DU Putri, PH Feng, CF Lin, SM Haryana, MHNE Soesatyo, KY Lee, CL Han.
Proteomic networks associated with tumor-educated macrophage polarization and cytotoxicity potentiated by heat-killed tuberculosis
Scientific Reports 12 (1), 6881
Abstract
Local administration of attenuated mycobacterium has been used as a cancer treatment adjuvant to re-boost patient immune responses with variable clinical outcomes. We aimed to clarify the impact of attenuated heat-killed tuberculosis (HKTB) on tumor-associated macrophages which play critical roles in shaping immunological regulation in the tumor microenvironment. Upon HKTB stimulation, both primary macrophages derived from the peripheral blood of healthy subjects and from lung cancer patients as well as THP1-derived classically activated macrophages (Ms) and tumor-educated macrophages (TEMs) were polarized into the proinflammatory phenotype, as characterized by increased expression cluster of differentiation 86. A quantitative proteomic analysis revealed that stimulated TEMs were unable to activate the toll-like receptor 2, signal transducer and activator of transcription 1, or nuclear factor-κB signaling. Instead, they showed distinct intercellular adhesion molecule 1 signaling, impaired cell adhesion, and mitochondrial dysfunction. These molecular mechanisms might contribute to lower cytotoxicity of HKTB-stimulated TEMs against A549 cells via the release of distinct inflammatory cytokines compared to HKTB-stimulated Ms. Our study provides an unbiased and systematic interpretation of cellular and molecular alterations of HKTB-reeducated macrophages which should help illuminate potential strategies of HKTB-stimulated macrophage-based combination therapy for cancer treatment.

 Total Users : 319332
Total Users : 319332
 Yuan-Feng Lin
Yuan-Feng Lin 